Lexogen 12 nt Unique Dual Index System
- 384 Unique Dual Indices cover all barcoding needs
- Rescue of reads maximizes data output and
saves sequencing costs - Universal sets with adjustable read-out length
accommodate each experiment scale - Optimized inter-index distances translate
into superior index error correction
Description
Lexogen 12 nt Unique Dual Index System (UDI) for RNA-Seq
Lexogen’s UDI 12 nt Unique Dual Indexing Sets feature superior error correction for maximal sequencing data output and are introduced at the PCR step of Lexogen’s library kits. Convenient bundles containing the 12 nt UDIs are available for:
- QuantSeq 3’ mRNA-Seq Library Prep Kit FWD for Illumina
- CORALL Total RNA-Seq Library Prep Kit for Illumina
- CORALL mRNA-Seq Library Prep Kit for Illumina
a) alternative Sets (A1 to A4, Cat. No. 101-104, 156) to the provided Set B1 in the following library prep kits:
- QuantSeq 3′ mRNA-Seq Library Prep Kit REV with UDI Set B1, Cat. No. 225
- LUTHOR HD 3′ mRNA-Seq Library Prep Kit with UDI Set B1, Cat. No. 221
b) Add-on Sets (A1 to A4 and B1, Cat. No. 101-104, 156, 105) in the following library prep kits:
- QuantSeq-Pool Sample-Barcoded 3′ mRNA-Seq Library Prep Kit, Cat. No. 139
- LUTHOR HD 3′ mRNA-Seq Library Prep Kit (no UDI), Cat. No. 204
- LUTHOR HD Pool 3′ mRNA-Seq Library Prep Kit, Cat. No. 205
Introduction
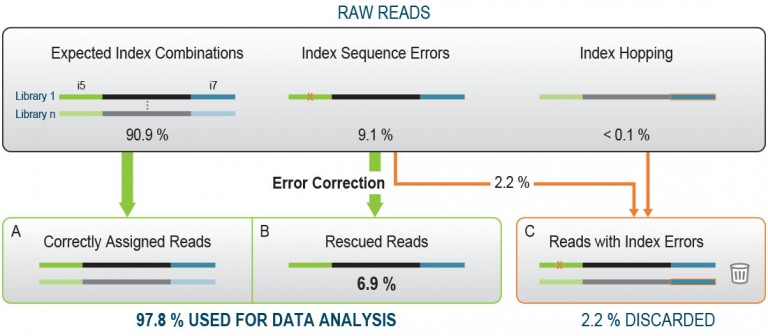
A critical consideration for any multiplexed RNA-Seq workflow is to avoid errors in the index read-out, which can result in the mis-assignment of sequencing reads to the wrong samples. While the majority of the raw reads will have the expected index combinations (Fig. 1A), read mis-assignment can occur on all Illumina platforms. This happens due to two main events: Index Hopping and random Index Sequence Errors.
During Index Hopping an index sequence of one library is incorrectly added to another library which may affect 0.1 – 2 % of all reads [1]. Only the use of Unique Dual Indexing (UDI), where each library in a given pool is barcoded with unique i7 and unique i5 index sequences, unambiguously identifies reads with hopped indices. Such reads are removed from downstream analysis and discarded (Fig. 1C).
Read mis-assignment due to random Index Sequence Errors occurs when an error in one index sequence transforms the index into another one that is present within the same multiplexed sample pool. UDIs resolve such mis-assignment and the read is discarded.
More frequently, an Index Sequence Error results in an index sequence that does not match any other index in the pool, and the read is initially classified as undetermined. If the index sequence in question is different enough from the other index sequences in this pool, then error correction can be applied to recover a significant share of these reads (4 – 7 % of the initial reads, Fig. 1B). The performance of this error correction depends predominantly on the quality of the index design, as deficient index design can result in a higher rate of faulty error correction. Due to their unique design the Lexogen UDI 12 nt Unique Dual Indices minimize the impact of Index Sequence Errors and enable maximal data output gain by error correction.
References
[1] Illumina, Effects of Index Misassignment on Multiplexing and Downstream Analysis (2017) 770-2017-004-D.
Universal Application
The Lexogen UDI 12 nt Unique Dual Indexing Add-on Kits are compatible with library prep kits for RNA and DNA sequencing from all vendors utilizing TruSeq™ – compatible stubby adapters (where partial Illumina adapters are introduced during the workflow and completed with the index information during the library amplification step).Superior Error Correction Maximizes Sequencing Yield
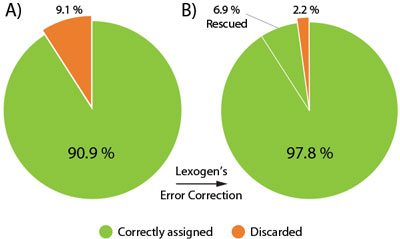
The Lexogen UDI 12 nt Unique Dual Indices are 12 nucleotides (nt) long and designed to maximize inter-index distance for different sample numbers and index read-out lengths. In a typical experiment using the full 12 nt index read-out around 9.1 % of the initial raw reads contain a random Index Sequence Error (Fig. 2A). This renders them undetermined, hence removing these reads from downstream analysis.
Lexogen’s advanced index design enables the rescue of 76 % of these undetermined reads (6.9% of the initial reads), even if multiple nucleotides of the index contain errors. The useful output thereby increases to 97.8 % of the initial reads, an unprecedented performance due to the cutting-edge index design (Fig. 2B).
Scalable Index Read-out Length
The design of Lexogen’s 12 nt UDIs enables scalable read-out lengths of 12, 10, and 8 nucleotides. The UDIs therefore support all kinds of requirements for multiplexing, which depend on experiment type, sequencing equipment, desired read depth, and / or the number of pooled libraries. For small sample sizes (e.g., 24 samples) short indices (e.g. 8 or 10 nt) are sufficient to ensure high accuracy and reliable error correction. For more than 96 samples however, 8 nt index read-out does not allow reliable error correction anymore, and 10 or 12 nt read-outs are required.
While needing slightly more sequencing cycles, 12 nt long index sequences also provide the ability to correct not only one but two (or in very small sets, three) Index Sequence Errors. Adjustable index read-out-length allows tuning your indexing needs to the experiment design, without the need to purchase separate indexing sets.
Nested Index Set Design for Highest Accuracy
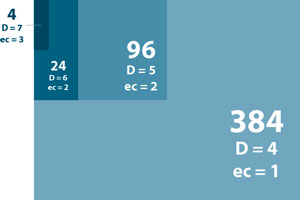
To provide optimal index subsets for these varying multiplexing needs, Lexogen has designed the 12 nt UDIs in a nested approach: Small subsets benefit from Lexogen’s nested index system by having the largest inter-index distance and highest error correction capacity while larger subsets provide for higher multiplexing needs.
Moreover, all subsets are nucleotide-balanced at each index position for optimized cluster identification in the NGS run. Using a proprietary algorithm, Lexogen has designed more than 9,216 UDIs (24x 384 subsets) with the capacity of correcting at least one error. Such sets with more than 384 UDIs are available upon request and enable extreme levels of multiplexing while still providing excellent error correction.
Conclusion
The Lexogen UDI 12 nt Unique Dual Index system adapts to the user’s needs while always providing highest inter-index distance and maximal error correction capacity. Read mis-assignment due to Index Hopping is avoided, and Index Sequence Errors can be corrected with highest accuracy. Thereby, the system provides the optimized indexing solution for current and future barcoding requirements.| Subset | 12 nt | 10 nt | 8 nt | |||
|---|---|---|---|---|---|---|
| D | ec | D | ec | D | ec | |
| 384 | 4 | 1 | 3 | 1 | 2 | 1* |
| └ 96 | 5 | 2 | 4 | 1 | 3 | 1 |
| └ 24 | 6 | 2 | 5 | 2 | 4 | 1 |
| └ 4 | 7 | 3 | 6 | 2 | 5 | 2 |
Table 1 | Comparison of distance and error correction capacity in Lexogen’s nested 12 nt UDI sets with 8, 10, and 12 nt read-out. Inter-index distance (D) and number of errors that can be corrected (ec) are compared for subsets of 384, 96, 24, and 4 libraries and the three possible read-out lengths. For smaller subsets (up to 96 samples) a read-out of 8 or 10 nt allows correction of one error and thus recovery of additional reads. Larger subsets require a read-out of 10 or 12 nt to benefit from the error correction. The ec values represent the number of all errors (including substitutions, insertions, and deletions) that can be confidently corrected, except for *. In this case error correction can only address substitutions.
FAQ
Frequently Asked Questions
Access our frequently asked question (FAQ) resources via the buttons below.
Please also check our General Guidelines and FAQ resources!
How do you like the new online FAQ resource? Please share your feedback with us!
Downloads
Lexogen 12 nt Unique Dual Index System (UDI) for RNA-Seq
New Version (Version 2):
Older Version (Version 1):
Safety Data Sheet
If you need more information about our products, please contact us through support@lexogen.com or directly under +43 1 345 1212-41.
Buy from our webstore
Need a web quote?
You can generate a web quote by Register or Login to your account. In the account settings please fill in your billing and shipping address. Add products to your cart, view cart and click the “Generate Quote” button. A quote in PDF format will be generated and ready to download. You can use this PDF document to place an order by sending it directly to sales@lexogen.com.
Web quoting is not available for countries served by our distributors. Please contact your local distributor for a quote.





